This is #4 in the series of how we can tolerate the pandemic in a lot of slow, steady progress and hard work. As a quick recap, the first post laid out a bit of a roadmap (March 23, 2020); the second (April 17, 2020) and third (June 26, 2020) noted updates on the categories described in the first.
In the two months since my previous post, one of the most exciting advances has been in improved understanding of the transmission dynamics of SARS-CoV-2, particularly with respect to aerosol-like transmission. An Aug 11 New York Times article has a good summary of some of the research in this area. Tl;dr: Ballistic (large droplet) transmission is probably still a big factor, and is helped quite a bit by masks. Aerosol transmission is a big contributor particularly to superspreader events (and particularly indoors). Cloth or surgical masks help but are not a silver bullet, particularly in crowded, confined spaces.
Why is this exciting? Because it helps us bring more tools to bear against transmission. It furthers the (already very strong) case for the outdoors being substantially safer than being indoors; it reinforces the importance of masks as part of a source control strategy; and it suggests that improvements to ventilation and good (HEPA or MERV-13 or higher) filtration also have a role to play in reducing the number of people who get infected from a transmitting individual.
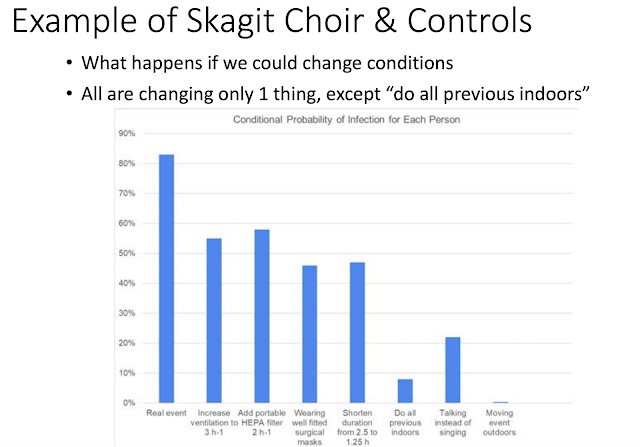
For more on this, I suggest starting with the Harvard Healthy Buildings report from June and this slide deck from CU Boulder. As a taste of some of the great things in there, this slide, analyzing the infection probability of a known real super-spreader event (an indoor choir practice) while changing different components of the scenario, such as increasing ventilation. Or just moving the darn thing outside, which would have approximately solved the problem. (The advantage of being outside was already clear as of the previous update in June, but more evidence and understanding of why is good.)
With that said, let's get back to the framework from the previous posts: Testing, PPE/Supplies, Treatments, and Societal changes to reduce contact. I'll fold ventilation improvements into the latter, since that's where I'd previously discussed things like plexiglass barriers in retail.
Testing
I'm going to just incorporate covidtracking's graph here, since it's now nicer than mine [
source]
So that's .. not what I'd like to see, but we have to be careful reading that. In update #3 (June), we were at 450k tests/day. As of now the US is up to about 700k. Better than June, but case counts are also still higher than they were in June; test positive rates are only just now getting back down to where they were. The good news is that we're testing a lot; the bad news is that with cases so high our testing isn't anywhere near sufficient to keep cases low.
PPE
N95 mask production continues to ramp up about as expected. 3M says it's on track to produce 95 million per month by October in the US, and 2B annually for the global market. In our previous update, 3m was trying to hit 50M/month by July, which they seem to have. Recall that DHS estimates we need about 300M masks/month in the US, but 3M isn't the only supplier. We're basically on track ramping up our PPE capacity, but it also still may be until the end of 2020 that we've really gotten this solved.
Anecdotally, N95 masks are showing up occasionally on Amazon now (before getting re-restricted for healthcare use only). Reusable options are emerging. But there are still few high-quality surgical masks (ASTM level 1-3 certified) available for the general public, which would be an improvement over the high variance in quality of uncertified masks.
The Purell Indicator
I've been monitoring availability of Purell as one interesting, informal indicator of the availability of hand sanitizer. In the last two weeks, it's become available again. There's been plenty of other-brand hand sanitizer available for the last two months, but as one of the most well-known brands, this is a good sign that manufacturing is catching up with demand.
Treatment Improvements
The big news two months ago was the outcome of the dexamethasone study; that remains part of the standard of care. Overall, studies of the infection fatality rate (IFR) suggest that it is lower now than in earlier phases of the epidemic, which is likely due to a combination of factors: treatment improvements, less overloading of hospitals, and better protection of highly-vulnerable populations such as nursing homes. But within this hides an important benefit: It's better to get SARS-CoV-2 today than it was in February. And it's likely to continue to improve as we run through additional options for improving treatment.
There are now
approximately 10 vaccine candidates in phase III trials. (I say approximately, because two of them are technically approved, but have been approved prior to their phase III trial, which means that their use will effectively be a phase III, albeit a potentially poorly controlled one). Prospects for the vaccine remain good:
- There are candidates based upon several different technologies.
- Results appear promising that infection yields protection for at least several months for most people.
The different technologies part is important. Several of the candidate vaccines are based upon an adenovirus vector, which has the drawback that prior infection by the same or similar adenovirus may reduce the vaccine's effectiveness. This
appears to be the case, for example, for the CanSino Ad5-vectored vaccine, which otherwise appears promising and is now in Phase III. This is in no way a fatal flaw for the Ad-based vaccines, but it means we may need alternatives. Fortunately, several of the other candidates are mRNA-based vaccines, such as that by Moderna in the US, which are likely to have entirely different failure patterns: we've never yet taken an mRNA vaccine to approval. But if one of these vaccines works, it probably won't fail for the same people for whom Ad5-vectored vaccines fail. And a few candidates, such as the vaccine from Wuhan Institute of Biological Products / Sinopharm, are inactivated virus vaccine, presenting a third mechanism to play with.
It's important to remember that no vaccine is likely to be perfect, and the deployment of a vaccine is not next week. Even once manufacturing is ramped up, several of the vaccines require two doses, potentially separated by several weeks. But progress remains good.
I'm not going to say much about convalescent plasma. Too much stupid politics going around about it, and it's still awaiting more randomized trial results. It's unlikely to be a silver bullet either, of course: making it requires people who're sick, it's expensive and rare, and its efficacy is as yet unknown. More scalable technologies, such as monoclonal antibodies,
are still in development & testing.
Societal and Environmental Changes
As noted above, this is where I'm most excited this month. Ventilation improvements to existing buildings can be expensive, but they pay dividends for years in healthier, more productive occupants, so upgrading as part of a pandemic-reduction strategy may not be a net cost increase as much as an unexpected cost shift to, well, now. This is something we can and should be pursuing aggressively, but we'll see how much traction it gets. I'd love to find someone/where that tracks ventilation system upgrades!
A large concern now is school reopening. We're just seeing the leading edge of it, with universities such as UNC
reopening and then backtracking on it. Many k-12 schools have delayed their reopening plans as cases in the US remain well above levels where opening would be safe. School closures have been widely regarded as one of the more effective NPIs (non-pharmaceutical interventions), both because they reduce the chances of children spreading the disease, but also because they reduce parents' mobility.



Comments
Post a Comment